Trimethylchlorosilane
Description
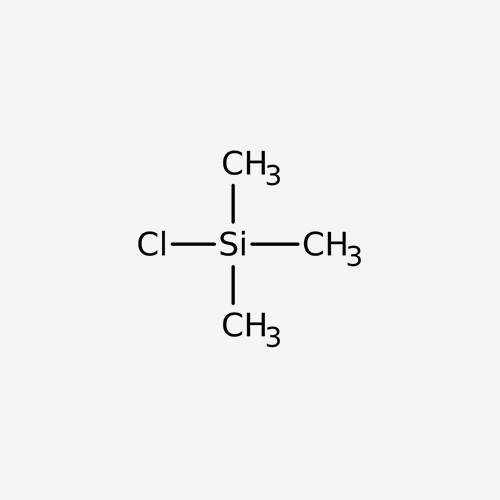
Chlorotrimethylsilane is a silyl chloride consisting of a central silicon atom covalently bound to one chloro and three methyl groups. Chlorotrimethylsilane is a derivatization agent used in gas chromatography/mass spectrometry applications. It has a role as a chromatographic reagent.
Trimethylchlorosilane appears as a colorless fuming liquid with a pungent odor. The vapor and liquid may cause burns. Vapors are heavier than air. It is also used in Remedesvir drug for Novel Coronavirus drug synthesis.





Category
Pharmaceutical Intermediates
CAS no
75-77-4
Purity
>=99%
Formula
C3H9SiCl
Synonyms
⦁Trimethylsilyl chloride
⦁
Chlorotrimethylsilane
⦁
TMCS
⦁
Monochlorotrimethylsilicon
⦁
Chloro(trimethyl)silane
Form
Liquid
Uses
⦁ In preparation of trimethyl halides, Aldols, pseudohalides, preparation of alkynes, esters, ketones, and aldehydes, also used as a catalyst to increase the reactivity of other silylation reagents. They are also used as a coating for silicon and glass surfaces and in the production of silicone (polysiloxane) polymers.