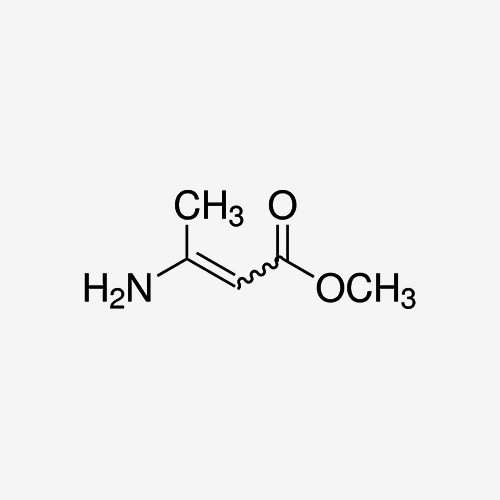
Introduction to Methyl 3-Aminocrotonate:
Methyl 3-Aminocrotonate, also known as 3-Aminocrotonic acid methyl ester, is a vital building block in organic chemistry. With the chemical formula C5H9NO2, this compound holds immense significance in the synthesis of pharmaceuticals, agrochemicals, and fine chemicals due to its unique structural features and reactivity.
Formula:
CH3C(NH2)=CHCOOCH3
Synonyms:
- Methyl 3-amino-2-butenoate
- 3-Amino-2-butenoic Acid Methyl Ester
Properties of Methyl 3-Aminocrotonate:
- Chemical Structure: Methyl 3-Aminocrotonate features a conjugated system comprising an amino group (-NH2) and a double bond (-C=C-) adjacent to the carboxyl group (-COOCH3). This structural arrangement contributes to its diverse reactivity and functional versatility.
- Physical Characteristics: Methyl 3-Aminocrotonate is typically found in the form of a colorless to pale yellow liquid with a characteristic odor. It exhibits moderate solubility in polar solvents such as water, ethanol, and acetone.
- Reactivity: The presence of both an amino group and an α,β-unsaturated carbonyl functionality makes Methyl 3-Aminocrotonate a valuable intermediate in various organic transformations, including Michael addition reactions, condensation reactions, and cyclization reactions.
- Stability: Under appropriate storage conditions, Methyl 3-Aminocrotonate maintains stability, allowing for extended shelf life and ease of handling in laboratory and industrial settings.
Applications of Methyl 3-Aminocrotonate:
Methyl 3-Aminocrotonate serves as a key intermediate in the synthesis of pharmaceutical compounds, particularly those with biological activity against a range of diseases. It is utilized in the preparation of medicinally important molecules such as beta-lactam antibiotics, antiviral agents, and amino acid derivatives.
The versatile reactivity of Methyl 3-Aminocrotonate makes it indispensable in the synthesis of agrochemicals, including herbicides, insecticides, and fungicides. Its incorporation into pesticide formulations enhances efficacy and environmental safety.
Methyl 3-Aminocrotonate finds applications in the production of specialty chemicals and intermediates for various industrial processes. It is employed in the synthesis of fragrances, flavors, and other high-value compounds.
In academic and industrial research laboratories, Methyl 3-Aminocrotonate serves as a valuable tool for exploring new synthetic methodologies and developing novel chemical entities with potential applications in diverse fields.
Benefits of Methyl 3-Aminocrotonate:
Versatility
Methyl 3-Aminocrotonate offers versatility in organic synthesis, enabling the construction of complex molecular architectures through diverse synthetic pathways.
Efficiency
Its selective reactivity and functional group tolerance streamline synthetic processes, leading to higher yields and reduced reaction times.
Customizability
Methyl 3-Aminocrotonate can be readily modified and functionalized to tailor its properties according to specific application requirements, providing flexibility in chemical design.
Quality Assurance
Our Methyl 3-Aminocrotonate undergoes rigorous quality control measures to ensure purity, consistency, and compliance with industry standards, thereby guaranteeing reliable performance in various applications.
Technical Support
Our team of experts is committed to providing technical assistance and guidance to customers, offering solutions to challenges and optimizing synthetic processes for maximum efficiency and cost-effectiveness.
Safety Considerations:


While Methyl 3-Aminocrotonate is a valuable synthetic intermediate, proper handling and safety precautions must be observed to mitigate potential risks. Key considerations include:
Personal protective equipment, including gloves, goggles, and lab coats, should be worn when handling Methyl 3-Aminocrotonate to prevent skin contact, eye irritation, and inhalation of vapors.
Work areas should be adequately ventilated to minimize exposure to airborne contaminants and ensure a safe working environment.
Methyl 3-Aminocrotonate should be stored in a cool, dry place away from heat, moisture, and incompatible materials. Proper labeling and segregation from other chemicals are essential to prevent cross-contamination.
Methyl 3-Aminocrotonate is a versatile compound with wide-ranging applications in organic synthesis, pharmaceuticals, agrochemicals, and fine chemicals. Its unique reactivity, combined with its customizable properties and reliability, makes it an indispensable tool for researchers and manufacturers striving for innovation and excellence in their respective fields.