Potassium HMDS
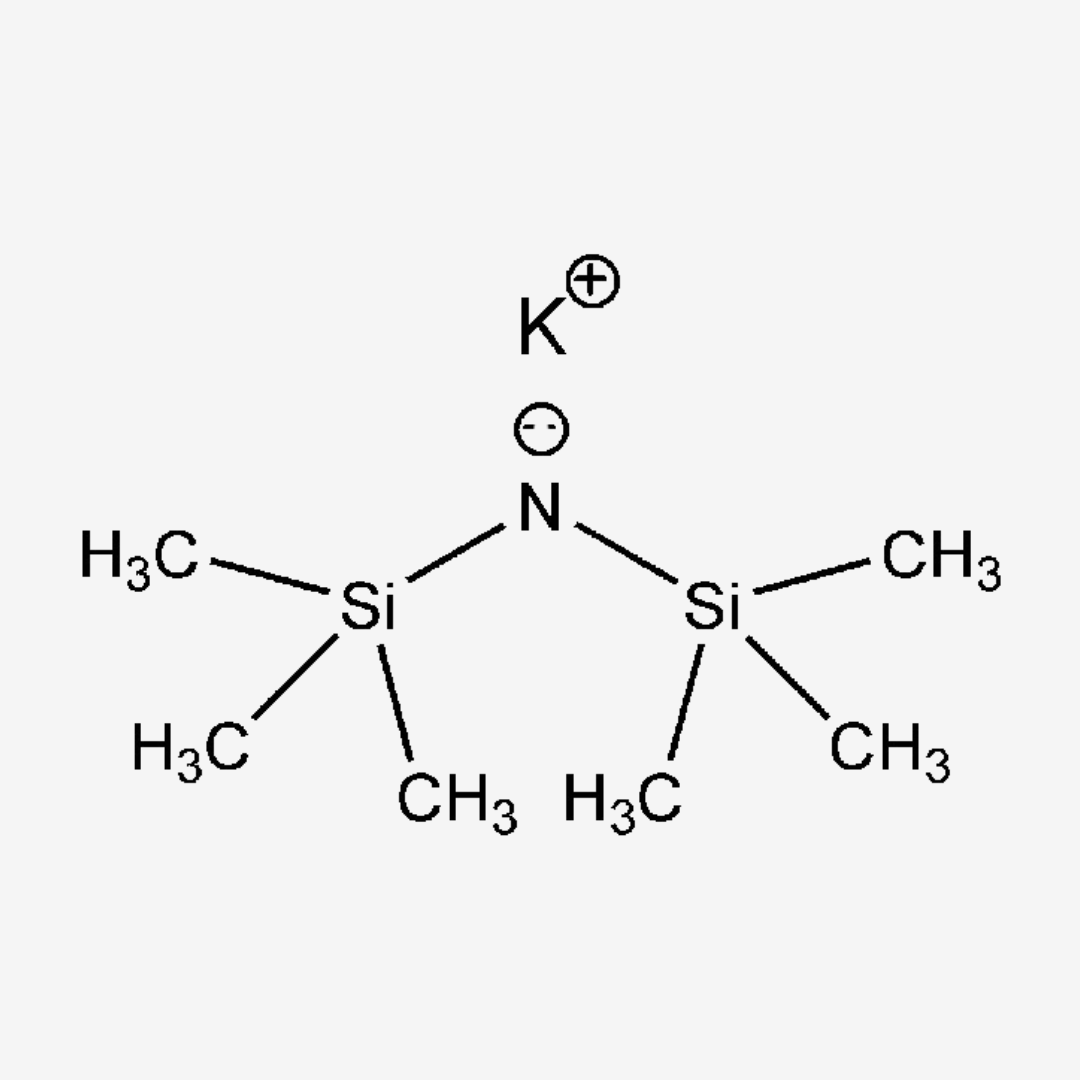
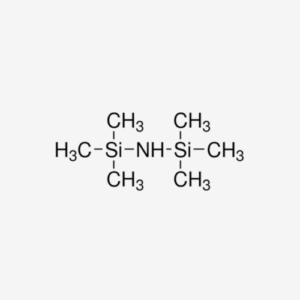
Potassium hexamethyldisilazide (KHMDS) is a potent base commonly used in organic synthesis as a strong non-nucleophilic base. With the chemical formula K+[(CH3)3Si]2N-, it plays a crucial role in various reactions due to its unique properties and reactivity.
Formula:
C6H18KNSi2
Synonyms of Potassium HMDS:
- Potassium hexamethyldisilazine
- Potassium bis(trimethysilyl) Amides
- KHMDS
Potassium HMDS
- Chemical Structure : KHMDS consists of a potassium cation (K+) and two hexamethyldisilazide anions [(CH3)3Si]2N-, providing strong basicity.
- Physical State : It is typically supplied as a solution in solvents such as tetrahydrofuran (THF) or toluene.
- Solubility : It is soluble in a variety of organic solvents, facilitating its use in solution-phase reactions.
- Stability : KHMDS is stable under inert atmosphere conditions but should be handled and stored carefully to avoid exposure to moisture or air.
- Reactivity: KHMDS is a strong non-nucleophilic base, making it suitable for deprotonation reactions and metalation reactions.
What are the Applications of Potassium HMDS:
KHMDS is commonly employed as a base for deprotonation reactions, such as the preparation of organolithium or Grignard reagents.
It serves as a powerful base for metalation reactions, enabling the formation of organometallic compounds from organic substrates.
KHMDS can be used in asymmetric synthesis as a chiral base or as a reagent in enantioselective transformations.
In certain cases, KHMDS can act as a catalyst or co-catalyst in organic reactions, enhancing reaction rates or selectivity.
It may be employed in polymerization reactions as a base or initiator for the synthesis of functionalized polymers.
What are the Benefits of Potassium HMDS:
Strong Basicity
KHMDS is a powerful non-nucleophilic base, enabling efficient deprotonation and metalation reactions.
Versatility
It finds utility in a wide range of organic transformations, making it a valuable tool in synthetic chemistry.
Selective Reactivity
KHMDS exhibits selective reactivity, allowing for precise control over reaction outcomes and product distributions.
High Yield
Its strong basicity and reactivity often lead to high yields and efficient transformations in organic synthesis.
Catalytic Potential
In addition to its role as a base, KHMDS may exhibit catalytic activity, broadening its scope of applications in organic chemistry.
What are the Safety Considerations for Potassium HMDS:

While KHMDS is a valuable reagent in organic synthesis, proper handling is essential to ensure safety:
KHMDS should be handled under inert atmosphere conditions, as it is sensitive to air and moisture.
Personal protective equipment such as gloves, goggles, and lab coats should be worn to prevent skin and eye contact.
It should be stored in tightly sealed containers under an inert atmosphere to prevent degradation and exposure to moisture or air.
Care should be taken to avoid spills and splashes, as KHMDS can cause skin and eye irritation upon contact.
Potassium hexamethyldisilazide (KHMDS) stands as a versatile reagent in organic synthesis, offering powerful basicity and selective reactivity. Its applications span across various domains of organic chemistry, enabling the synthesis of complex molecules and materials. By harnessing the potential of KHMDS, researchers and chemists can explore new avenues in chemical synthesis and catalysis, driving innovation and discovery in the field of organic chemistry.
We Provide Best in class service and on time delivery to fulfill the requirement of this product worldwide.